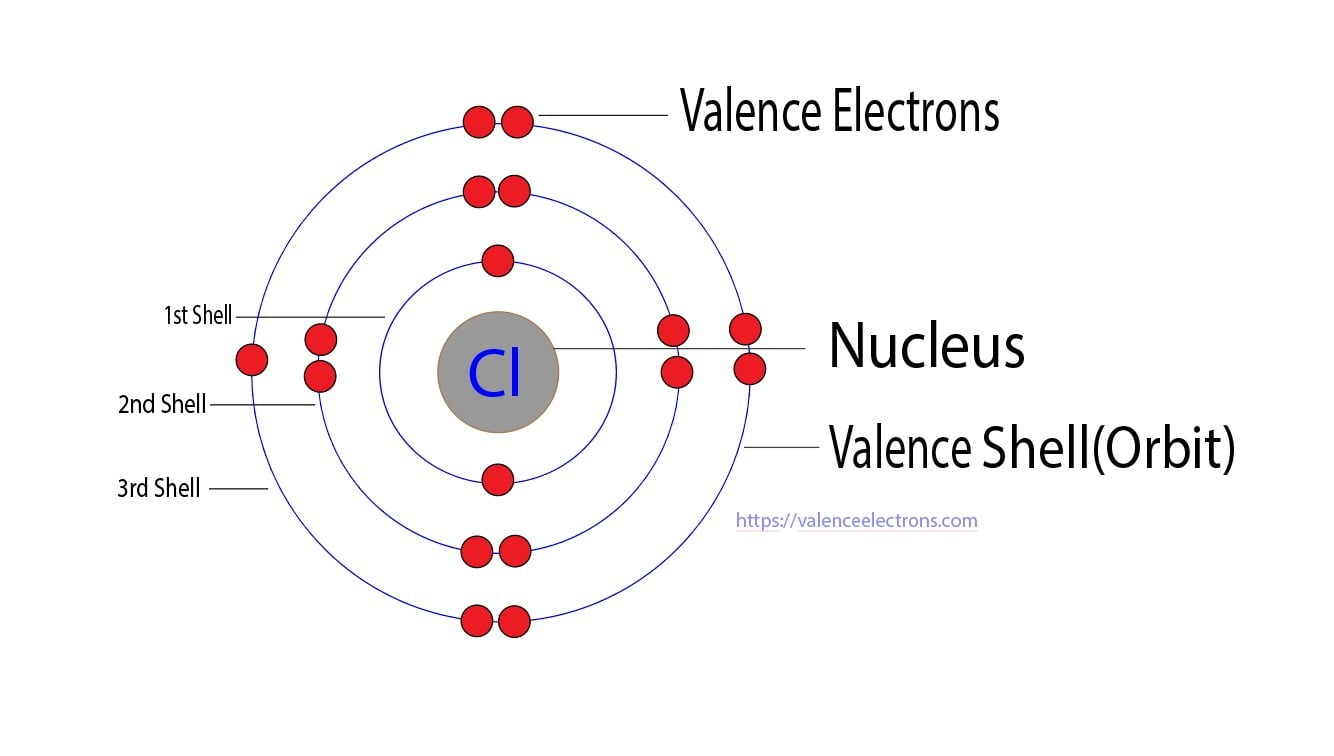
An Atom Of Chlorine Has How Many Valence Electrons . chlorine is in group viia (group 17), so it would have seven valence electrons. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. chlorine has 7 valence eletrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). The most stable atoms have a full valence shell of electrons. You know this by looking at the groups (vertical) of the. valence electrons are the electrons in the outermost shells. how many valence electrons does chlorine have? The electron configuration of chlorine is. Calcium would have two valence electrons, since it is in group iia (group 2). group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. chlorine has 7 valence electrons.
from valenceelectrons.com
Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. valence electrons are the electrons in the outermost shells. chlorine has 7 valence electrons. You know this by looking at the groups (vertical) of the. The most stable atoms have a full valence shell of electrons. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. chlorine is in group viia (group 17), so it would have seven valence electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). chlorine has 7 valence eletrons. The electron configuration of chlorine is.
How Many Valence Electrons Does Chlorine (Cl) Have?
An Atom Of Chlorine Has How Many Valence Electrons Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). valence electrons are the electrons in the outermost shells. chlorine is in group viia (group 17), so it would have seven valence electrons. The most stable atoms have a full valence shell of electrons. how many valence electrons does chlorine have? chlorine has 7 valence electrons. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. You know this by looking at the groups (vertical) of the. Calcium would have two valence electrons, since it is in group iia (group 2). The electron configuration of chlorine is. chlorine has 7 valence eletrons.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in An Atom Of Chlorine Has How Many Valence Electrons The electron configuration of chlorine is. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. chlorine has 7 valence eletrons. how many valence electrons does chlorine have? there are. An Atom Of Chlorine Has How Many Valence Electrons.
From exozjrgwj.blob.core.windows.net
Lewis Dot Structure For Chlorine Atom at Ernest Silverstein blog An Atom Of Chlorine Has How Many Valence Electrons The most stable atoms have a full valence shell of electrons. valence electrons are the electrons in the outermost shells. chlorine is in group viia (group 17), so it would have seven valence electrons. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. You know this by looking at the groups (vertical) of the. Calcium. An Atom Of Chlorine Has How Many Valence Electrons.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons An Atom Of Chlorine Has How Many Valence Electrons The most stable atoms have a full valence shell of electrons. The electron configuration of chlorine is. chlorine has 7 valence electrons. valence electrons are the electrons in the outermost shells. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. Calcium would have two valence electrons, since it is in group iia (group 2). You. An Atom Of Chlorine Has How Many Valence Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons An Atom Of Chlorine Has How Many Valence Electrons chlorine has 7 valence electrons. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. Calcium would have two valence electrons, since it is in group iia (group 2). chlorine has 7 valence eletrons. valence electrons are the electrons in the outermost shells. group 17 elements, including fluorine and chlorine, have seven electrons in. An Atom Of Chlorine Has How Many Valence Electrons.
From www.slideserve.com
PPT 3 rd Orbitals PowerPoint Presentation, free download ID2062267 An Atom Of Chlorine Has How Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). how many valence electrons does chlorine have? chlorine is in group viia (group 17), so it would have seven valence electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). You know this by looking at. An Atom Of Chlorine Has How Many Valence Electrons.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image An Atom Of Chlorine Has How Many Valence Electrons chlorine is in group viia (group 17), so it would have seven valence electrons. The most stable atoms have a full valence shell of electrons. Calcium would have two valence electrons, since it is in group iia (group 2). You know this by looking at the groups (vertical) of the. Because chlorine has an electronic configuration 1s 2 2s. An Atom Of Chlorine Has How Many Valence Electrons.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure An Atom Of Chlorine Has How Many Valence Electrons chlorine has 7 valence eletrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). how many valence electrons does chlorine have? The electron configuration of chlorine is. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. Calcium would have two valence electrons, since it is in group. An Atom Of Chlorine Has How Many Valence Electrons.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence An Atom Of Chlorine Has How Many Valence Electrons there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). how many valence electrons does chlorine have? chlorine has 7 valence electrons. valence electrons are the electrons in the outermost shells. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. Calcium would have two valence electrons, since. An Atom Of Chlorine Has How Many Valence Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram An Atom Of Chlorine Has How Many Valence Electrons there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). The electron configuration of chlorine is. chlorine has 7 valence eletrons. chlorine is in group viia (group 17), so it would have seven valence electrons. You know this by looking at the groups (vertical) of the. valence electrons are the. An Atom Of Chlorine Has How Many Valence Electrons.
From animalia-life.club
Lewis Dot Structure For Chlorine An Atom Of Chlorine Has How Many Valence Electrons The most stable atoms have a full valence shell of electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). chlorine has 7 valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). valence electrons are the electrons in the outermost shells. group. An Atom Of Chlorine Has How Many Valence Electrons.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library An Atom Of Chlorine Has How Many Valence Electrons chlorine has 7 valence eletrons. chlorine has 7 valence electrons. You know this by looking at the groups (vertical) of the. The electron configuration of chlorine is. how many valence electrons does chlorine have? valence electrons are the electrons in the outermost shells. there are a total of seven electrons present in the valence shell/outermost. An Atom Of Chlorine Has How Many Valence Electrons.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions An Atom Of Chlorine Has How Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. how many valence. An Atom Of Chlorine Has How Many Valence Electrons.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule An Atom Of Chlorine Has How Many Valence Electrons how many valence electrons does chlorine have? chlorine has 7 valence electrons. The electron configuration of chlorine is. chlorine is in group viia (group 17), so it would have seven valence electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Because chlorine has an electronic configuration 1s 2. An Atom Of Chlorine Has How Many Valence Electrons.
From www.shutterstock.com
585 Chlorine electrons 이미지, 스톡 사진 및 벡터 Shutterstock An Atom Of Chlorine Has How Many Valence Electrons The electron configuration of chlorine is. how many valence electrons does chlorine have? group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. Calcium would have two valence electrons, since it is in group iia (group 2). chlorine has 7 valence eletrons. The. An Atom Of Chlorine Has How Many Valence Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) An Atom Of Chlorine Has How Many Valence Electrons how many valence electrons does chlorine have? The most stable atoms have a full valence shell of electrons. You know this by looking at the groups (vertical) of the. The electron configuration of chlorine is. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). group 17 elements, including fluorine and. An Atom Of Chlorine Has How Many Valence Electrons.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk An Atom Of Chlorine Has How Many Valence Electrons chlorine has 7 valence electrons. You know this by looking at the groups (vertical) of the. valence electrons are the electrons in the outermost shells. chlorine is in group viia (group 17), so it would have seven valence electrons. The most stable atoms have a full valence shell of electrons. chlorine has 7 valence eletrons. . An Atom Of Chlorine Has How Many Valence Electrons.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons An Atom Of Chlorine Has How Many Valence Electrons The most stable atoms have a full valence shell of electrons. how many valence electrons does chlorine have? Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. chlorine is in group viia (group 17), so it would have seven valence electrons. chlorine has 7 valence eletrons. group 17 elements, including fluorine and chlorine,. An Atom Of Chlorine Has How Many Valence Electrons.
From circuitetappesu.z4.web.core.windows.net
Atomic Diagram Of Chlorine An Atom Of Chlorine Has How Many Valence Electrons Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). The electron configuration of chlorine is. You know this by looking at the groups (vertical) of the. how. An Atom Of Chlorine Has How Many Valence Electrons.